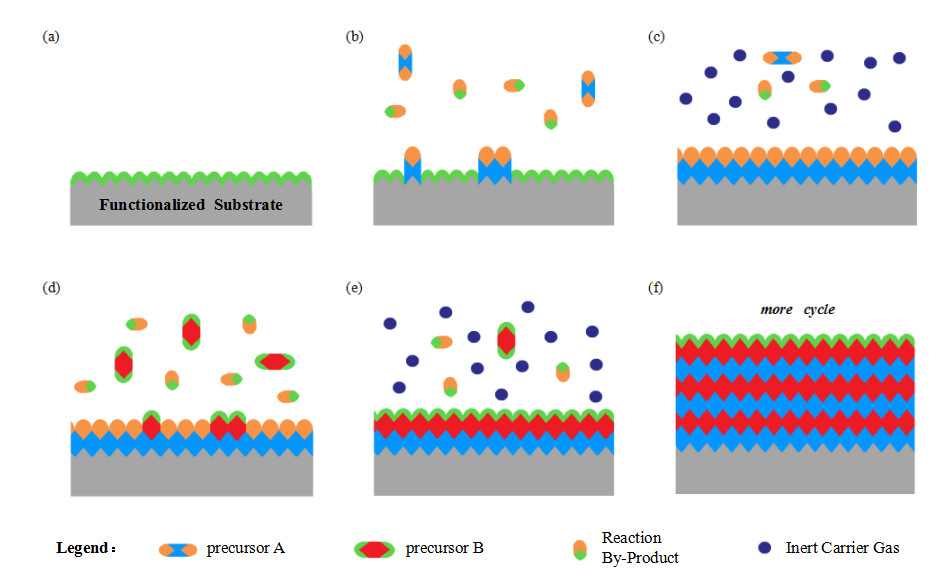
MEMS [22-25] is one of the critical technologies for the development of integrated circuits. It experienced rapid development since the 21st century, and has shown a strong vitality in a number of interdisciplinary areas. Thin film deposition technology, especially ALD technology, is an important process in MEMS surface micro-machining technology. Due to the thin film thickness and complex substrate structure, some models that are more suitable for processing small size are also proposed. Two models suitable for ALD thin film growth and post-deposition pattern prediction are described below.
(1) Cellular Automata Model
Cellular automata (CA) [24, 26-28] is a time-space discrete dynamic system. Each cell has a fixed grid representation and a finite discrete state. According to the same evolution rules, the whole cell is updated when time and local environment change. Due to its simple dynamic system composition, it has the advantage of high precision in three-dimensional space simulation. Cellular automata model is a kind of complex and changeable model without fixed mathematical formula. Among the numerous cellular automata models based on different starting points, the most influential one is the cellular automata classification based on dynamic behavior made by S.Wolfram [27] in the early 1980s, and the cellular automata classification based on dimension is also the simplest and most commonly used classification. For the treatment of thin film growth problem, this model shows its advantages of simplicity and matching with the growth model.
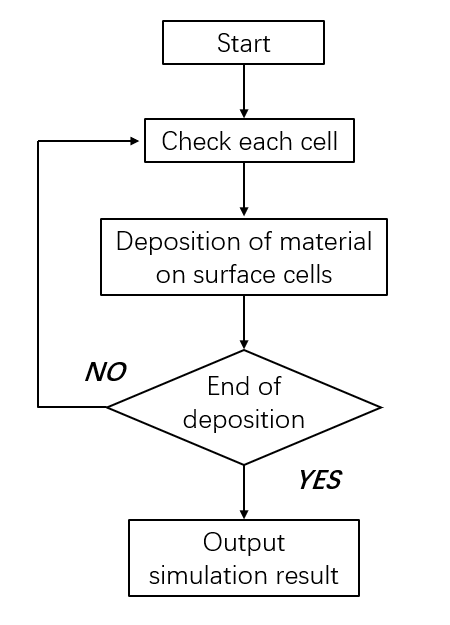
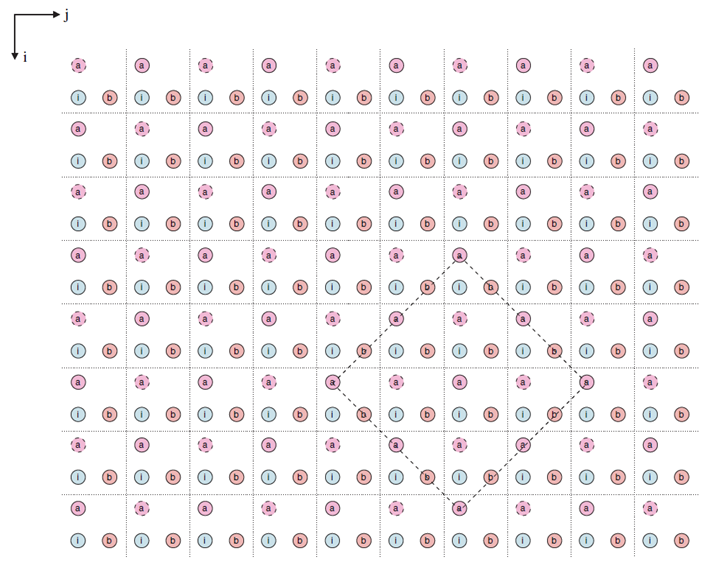
Cellular automata can be divided into the following four methods: Ordinary-CA method, Random-CA method, Continuous-CA method, and Dynamic-CA method. The Ordinary-CA method assumes that there are only two fixed states of atoms on the lattice, and only the principal direction rate is considered in the analysis. The Random-CA method was proposed by Than and Buttgenbach [29] et al., and whether the pre-deposited atom completed the opportunity process depends on a probability parameter P. The model also considers the interaction and connection state between atoms and adjacent atoms, and the probability P can express the deposition influence from different directions. Although this method can deal with the deposition rate in all directions, it is not clear about the boundary and the surface. The Continuous-CA method was proposed by Zhu and Liu [30, 31] et al., where a parameter M is used to represent the continuous state of each atom, and M can be randomly distributed between the two states. In this way, the influencing factors in the Random-CA model can be reduced, the simulation accuracy can be improved, and the surface of simulation results can be no longer fuzzy. Finally, the Dynamic-CA model is proposed by scholars such as Than and Buttgenbach [32], which mainly deals with the behavior of all atoms in the original model and only deals with the atoms related to the deposition surface, greatly improving the computing speed. According to the basic steps of cellular automata method, the computer can carry out sedimentation simulation according to the process in Figure 4.
Figure 4.
Flow chart of cellular automata. (2) Geometric Modeling
The idea of geometric modeling is to treat the sedimentary surface as a continuous whole [24, 33]. The shape of the sedimentary surface changes according to the geometric rules at certain time steps, and the sedimentary results are related to the geometric rules. Currently, there are about three geometric rules [34-36]: wulff-jaccodine rule proposed by Jaccodine, Slowness rule proposed by Sequin, and e-shapes rule proposed by Hubbard et al. The first rule applies the concept of "plane wave". The current contour surface will move along the normal vector method of contour surface with the growth rate calculated by the process, and will be updated with the number of cycles. The second rule applies the "slow rate" to calculate the trajectory change of the sedimentary surface. The "slow rate" is the reciprocal of the relative rate. In this way, it has a more detailed representation for the edge corner of the spit signal. The third rule introduces the "E vector", which is defined as the vector formed by two adjacent tangent points from the initial point to each unit of time. It combines the characteristics of the above two rules and improves the precision. The use of geometric methods requires that the rate of structural change in the deposition process must be known, and the precision of the model is also determined by the accuracy of the calculation of the rate of change.
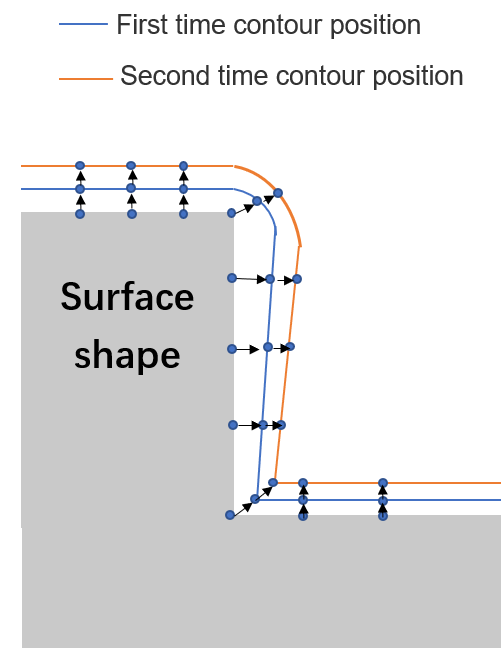
The model can be divided into Line algorithm and Level Set algorithm. Line algorithm is a kind of surface movement algorithm, which discretize the sedimentary surface into equidistant points and calculate the normal vector direction (sedimentary direction) of the point through the relation between the point and the adjacent position. The sedimentary distance is the product of the sedimentary rate and time. After each point changes with the time step, all points are connected with smooth lines in order, which can be expressed as the contour lines of the sedimentary structure [24]. The schematic diagram of line algorithm variation is shown in Figure 5. Level Set algorithm using collective concept, will represent for the curve or surface movement over time than one-dimensional function of zero Level Set function, Level Set, as long as in the process of calculation of the Level Set function derivation on both sides, you can get a partial differential equation, the solution of equation can get evolution after the Level Set function, according to the Level of machine before and after the change, get the movement curve or surface. This model has the advantages of high precision and high efficiency, and can deal with the change of topological form well.
Figure 5.
Schematic diagram of line algorithm. 2.2.3. Multi-scale Modeling of Atomic Layer Deposition
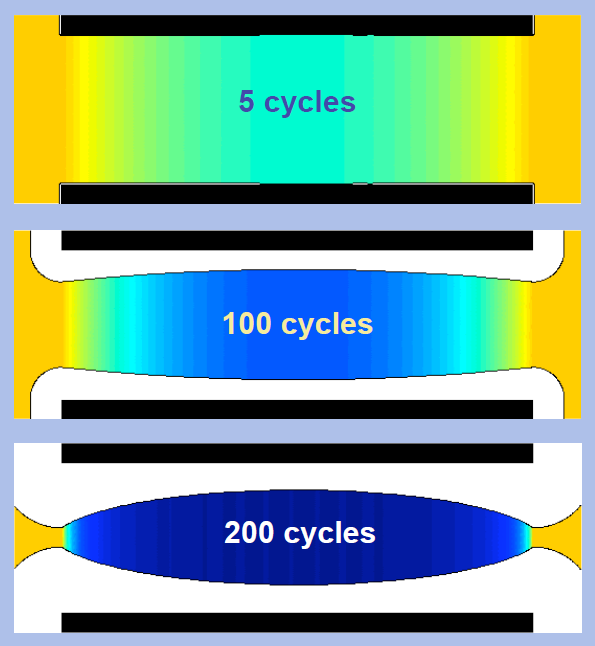
Multi-scale simulation model is a method to study the same problem from different angles. All the methods to solve problems have their incompleteness and limitations. In some "blind areas", other more effective and more accurate methods are used to solve local problems. At last, the whole idea of coordinating the whole situation is used to form a more comprehensive whole to solve problems. This is where the multi-scale model comes in. ALD modeling is suitable for this approach. ALD is a dynamic process with multiple time characteristics, and the time of molecular events within each reaction cycle is relatively fast, while nucleation and stable growth and other processes need a relatively long time to complete. Moreover, its length scale is not uniform, the gas before reaching the surface tends to go through a distance of 100 microns or longer, and the film formed is only the level of angstrom. So you need to split the problem and finally couple it. For example, Raymond A. Adomaitis [37, 38] applied the idea of multi-scale modeling when he studied the deposition of thin films in micron diameter tubular structures with ALD technology and studied the deposition rates in the middle and both sides of the tubes. This model can predict the difference of deposition rates in different positions of the tubes in different cycles, as shown in Figure 6. From the results of simulated deposition, this modeling method has high precision and is suitable for the prediction of post-deposition pattern in ALD process.
Figure 6.
Results of tube atomic layer deposition were simulated in different cycles [38].